Analytical Laboratory Services Market, By Public Health Organization: Types of Services
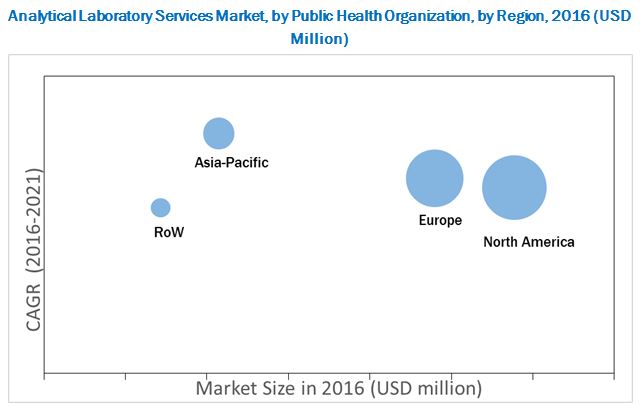
According to the new market research report “Analytical Laboratory Services Market by Public Health Organization, - by types of services (Stability, Raw Material, Physical Characterization, Method Validation, Microbial Testing, Environmental Monitoring, Bioanalytical Testing) - Forecast to 2021", published by MarketsandMarkets™, The global analytical laboratory services market, by public health organization is projected to reach USD 333.8 Million by 2021 from USD 202.8 Million in 2016, at a CAGR of around 10.5% during the forecast period.
Major Key Trends
The key trends for increase in market, by public health organization are the growing expenditure on drugs and medical devices by public health organizations, steps by the government to build up strong analytical testing abilities, increasing number of drug approvals and clinical trials, and rising demand for specialized analytical testing services. However, complex and innovative pharmaceutical products requiring a distinctive analytical testing approach is the major challenge hampering the growth of government support.
On the basis types of services
On the basis of type of service, the spend assessment is segmented into eight segments, namely, bioanalytical testing, batch release testing, stability testing, raw material testing, physical characterization, method validation, microbial testing, and environmental monitoring. In 2015, the bioanalytical testing segment accounted for the largest share of the analytical laboratory services market, by public health organization. This growth can be attributed to factors such as the usage and development of a large number of macromolecules and biosimilars for various therapeutic areas and the growing biopharmaceutical industry across the globe. The spending on batch release testing services is expected to account for the second largest share during the forecast period. The growth can be attributed to the need for checking and validating the process for product development among pharmaceutical & biopharmaceutical companies and the increasing usage of dissolution test in the development and approval of generic solid oral dosage forms.
To know more Speak to Analyst @ https://www.marketsandmarkets.com/speaktoanalystNew.asp?id=65590498
Comments
Post a Comment